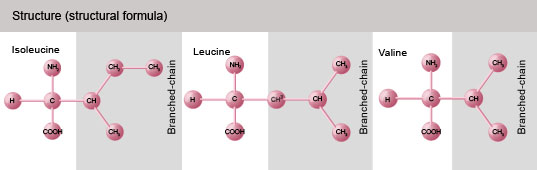
What are branched chain amino acids?
Amino acids are the building blocks of protein. Branched chain amino acids (BCAAs) are so called because of their structure, which includes a “side chain” of one carbon atom and three hydrogen atoms. There are three BCAAs: leucine, isoleucine, and valine. Of these, leucine is the most heavily researched, and appears to offer the biggest physiological benefit. (More on that below.)
For the science geeks, these hydrophobic (water-fearing) amino acids are referred to as “aliphatic” (from the Greek aleiphar, or oil), as their central carbon attaches to a branched non-cyclic, open carbon chain.
BCAAs provide the basis for protein synthesis and energy production (Harper AE et al 1984; Patti ME et al 1998; Xu G et al 1998; Anthony JC et al 2001). In fact, BCAAs can comprise up to one-third of muscle protein (Mero 1999). Because of their prevalence and involvement in protein synthesis and energy production, BCAAs are important to many metabolic processes.
However, if BCAAs are going to participate in these processes, they must be available to the body. This means we have to eat enough BCAAs, and at the right times, to enable such processes to occur.
Why is adequate BCAA intake so important?
The BCAAs are the only amino acids not degraded in the liver. All other amino acids are regulated by the gut and the liver before being circulated elsewhere in the body. However, BCAAs head directly into the bloodstream. This means that dietary intake of BCAAs directly influences plasma levels and concentrations in muscle tissue (Layman DK 2003). Interestingly, BCAAs are burned for energy (oxidized) during exercise, so they’re also an important exercise fuel.
Consuming BCAAs before training can increase uptake into muscle tissue (Mittleman KD et al 1998). This has many benefits:
- BCAA supplementation may lower lactate levels after resistance training and improve muscular oxidation.
- BCAAs may increase growth hormone (GH) circulation, which may be related to anabolic mechanisms causing muscle growth (De Palo EF et al 2001).
- BCAA supplementation may decrease serum concentrations of the intramuscular enzymes creatine kinase and lactate dehydrogenase following prolonged exercise. This can decrease muscle damage and improve recovery (Coombes JS, McNaughton LR 2000).
Muscle is an important site of BCAA activity. There is an increased cell concentration and breakdown of BCAAs in muscle tissue (Layman DK 2003). BCAAs are continuously released from the liver and other internal organs to skeletal muscle so that the BCAAs can assist in maintaining blood sugar levels. Indeed, BCAAs may be responsible for up to 40% of blood sugar production during exercise (Ahlborg G et al 1974; Ruberman NB 1975; see also Layman DK 2003).
What you should know
Because BCAAs are so important to muscle tissue, and because they help maintain blood sugar levels, it’s important to get enough to support your workouts. Consuming a carbohydrate, protein, and amino acid beverage during and after training can induce an insulin response, which helps transport BCAAs into cells. However, availability of leucine is more important than insulin. Within the muscle cell there’s one particular regulatory pathway for protein synthesis that’s stimulated by insulin, but dependent on leucine (Anthony et al 2000). In other words, protein synthesis (and hence muscle rebuilding) depends on how much leucine is available. And since BCAA levels decline with exercise, it makes sense to supplement with them during and/or after workouts (Mero 1999).
Because it’s so important to have leucine available for protein synthesis, if you train in a fasted state, or don’t eat after exercise, you’re going to lose more protein than you rebuild. However, if you eat adequate BCAAs during this time, especially leucine, you’ll enhance protein synthesis.
For extra credit
For the body to make new proteins, it needs an estimated daily leucine intake of between 1 to 4 grams/day (FAO/WHO/UNU 1985). That minimum intake needs to be met before leucine will be able to impact the insulin signaling pathway. But that’s just a baseline. Actual metabolic use, especially by athletes and people doing heavy resistance training, may be upwards of 12 grams/day.
There is a theory that BCAAs can limit central fatigue with endurance athletes, but it doesn’t appear to be supported by current data.
BCAA content of foods (grams of amino acids/100 g of protein)
Whey protein isolate 26%
Milk protein 21%
Muscle protein 18%
Soy protein isolate 18%
Wheat protein 15%
Source: USDA Food Composition Tables
Summary and recommendations
BCAAs play an important role in:
- Synthesis of proteins in general
- Glucose homeostasis (i.e. keeping blood sugar levels constant)
- Direct regulation of muscle protein synthesis (via insulin signaling cascade)
BCAAs’ potential impact on the aforementioned processes depends upon availability and dietary intake.
Adequate consumption of BCAAs may help manage body fat, spare muscle mass, and regulate glucose/insulin balance.
How can you put this knowledge to use?
Try adding BCAAs into your workout drink at a rate of 5 g BCAA per hour of training.
During periods of lower calorie intake, try adding a BCAA supplement every 2-4 hours during the day.
Eat, move, and live… better.©
Yep, we know… the health and fitness world can sometimes be a confusing place. But it doesn’t have to be.
Let us help you make sense of it all with this free special report.
In it you’ll learn the best eating, exercise, and lifestyle strategies — unique and personal — for you.
Click here to download the special report, for free.
References
Click here to view the information sources referenced in this article.




Share