Ever wonder what makes you hungry? Why some foods look more appealing than others? Why you’re always hungry for dessert? Or why you might open the fridge full of food, then stand there and say, “We have nothing good to eat!”?
The body is not a simple container of calories that can be added and subtracted. We’re driven by a complex play of chemicals that orchestrate food intake, desire, and food associations.
Appetite is our desire to eat. It’s controlled by a complicated interaction of hormonal signals that originate from fat cells, cells of the pancreas and cells in the gut. These signals are also processed through cognitive and emotional filters.
For example, if you ask a North American, “What’s comfort food?” they might say “macaroni and cheese”. Pretty sure you wouldn’t get the same answer in, say, Mongolia or Rwanda.
The foods we crave are a product of physiology and psychology. Appetite is different from hunger. Hunger is our physical need to eat. You can want to eat but not need to eat (for example, wanting to eat dessert after a big meal). Or you can need to eat but not want to eat (for example, losing your interest in food when you’re stressed).
How does this all work? And who’s driving the bus?
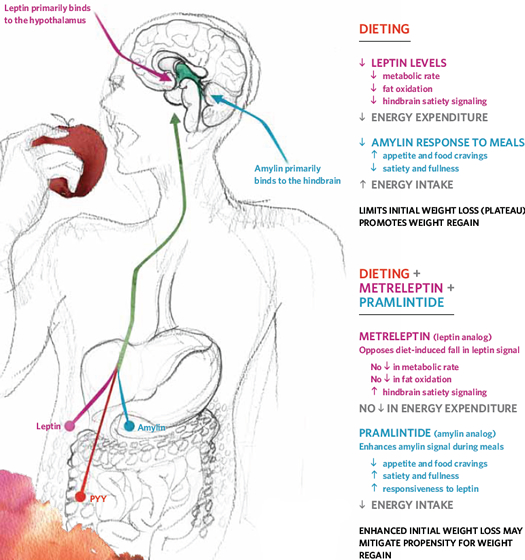
For anyone who has purposefully controlled food intake to lose weight, they know how powerful counter-regulation can be. Much of this seems to be mediated, or shaped by, our neuro-endocrine system, aka the interaction between our brains and our hormones.
When we lose stored fat, our body mounts a major response to conserve energy and boost appetite, defying further weight loss and encouraging regain.
Why is appetite regulation important?
If we under- or over-eat, problems arise. We can become malnourished, obese, fail to repair, lose reproductive ability and/or develop diseases.
For those who want to decrease body fat, a conscious restriction of energy intake is generally unsuccessful (more than 90% of the time weight is regained – and then some).
On the other hand, some people are successful at losing fat. Why does the first group fail and the second group succeed?
Finding the master controls
One of the best ways to grasp the importance of appetite regulation is to knock it out. In other words, the best way to learn what a hormone/gland does is to get rid of it and see what happens.
For instance, a simple defect in the hypothalamus, located in the brain, might mean someone may eat or starve themselves to death, like with Prader-Willi Syndrome. This tells us that the hypothalamus has a big role to play.
In fact, the hypothalamus is the ringmaster of appetite, but there are lots of acts in the circus. Major players in appetite regulation include insulin, thyroid hormone, glucagon like peptide-1 (GLP-1), endocannabinoids and cortisol. If any of these get out of whack, you can lose your life, literally.
We’re still learning about new pathways and chemicals involved in appetite, but below is a list of what we’ve got so far.
Orexigenic means appetite-stimulating, while anorexigenic means appetite-suppressing. (Both of these come from the Greek root orexis, or desire/appetite — notice here how “wanting to eat” is important.) Simple, huh? Not.
What you should know
Appetite is governed by two organ systems of the body, the endocrine system and the nervous system — their connection is sometimes known as the “neuroendocrine system”.
The endocrine system & appetite
Quick — what’s the largest endocrine organ in the body?
You might be surprised: it’s the GI tract. Yep, your gut is the biggest hormone player on the block. It produces and processes all kinds of hormones ranging from neurotransmitters to anabolic storage hormones to sex hormones.
The organs of the endocrine system are sensitive to changes in the body, and, in response to these changes, send out messengers (called hormones) to tell the body how to respond. These energy regulating hormones are classified into either short term or long term.
The vagus nerve is the key connection between the gut and the brain.
Various hormones play a role in appetite regulation and energy balance, including:
| Hormone | Origin and role |
| Calcitonin |
|
| Amylin |
|
| GLP-1 |
|
| Leptin |
|
| Gastrin |
|
| Secretin |
|
| Cholecystokinin (CCK) |
|
| Gastric inhibitory polypeptide (GIP) |
|
| Motilin |
|
| Somatostatin |
|
| PYY 3-36 |
|
| Ghrelin |
|
The nervous system & appetite
The nervous system acts via nerve impulses and neurotransmitters (hormone-like chemicals), directing nervous tissues, smooth muscles, and other organs of the body to move, mix, and propel foodstuffs that enter the digestive system.
While some appetite control originates from nervous and hormonal connections between the digestive system and the brain, the digestive system possesses its own, localized nervous system, referred to as the enteric nervous system.
It’s the “mini-brain” located in your gut. In this mini-nervous system, neurotransmitters are released, which can relay, amplify and modulate different signals between cells of the body.
Some of the neurotransmitters involved with appetite regulation include:
| Neurotransmitter | Role |
| Endocannabinoids | These participate in glucose and insulin metabolism in muscle and fat tissues. When endocannabinoid receptors are blocked, insulin sensitivity is improved. This can lead to less food intake and fat mass. When food intake is decreased, there seems to be an upregulation of endocannabinoid receptors and serious hunger is soon to follow (think: smoking weed and making a Taco Bell run). It seems that a diet with lots of omega-6 fats can promote endocannabinoid production, while a diet higher in omega-3 fat can inhibit it. Researchers are trying to develop endocannabinoid receptor blockers for humans. |
| Gamma aminobutyric acid (GABA) | GABA can act as an excitatory or inhibitory neurotransmitter depending on which cell receptor it binds to. The chief role of GABA is to stimulate GI motility and contribute to GI wall mucosal function. |
| Norepinephrine | Decreases digestive activity, which makes sense during fight or flight situations. When immediate, decisive, or aggressive action is required, digestion is a low priority. Stress not requiring immediate fight or flight type responses (such as deadlines, relationship challenges, etc.) also provokes norepinephrine release and this can impair digestive function. |
| Acetylcholine | In the digestive system, this neurotransmitter is responsible for stimulating digestive activity. It acts to stimulate smooth muscle contractions in the digestive organs and help move food through the GI tract. It also stimulates the release of other digestive hormones, dilates blood vessels, and increases intestinal secretions. It runs counter to the actions of norepinephrine. |
| Neurotensin | As dietary fat reaches the last section of the small intestine, cells located in the intestinal walls release neurotensin. It relaxes the lower esophageal sphincter, blocking the release of stomach acid and pepsin to regulate GI contractions. |
| Neuropeptide Y (NPY) | This neurotransmitter is present in both the brain and the enteric nervous system. In the brain, its action is to stimulate hunger and food intake while discouraging physical activity. Working in conjunction with leptin and corticotropic releasing hormone, this neurotransmitter plays a role in metabolism and body composition. It’s typically released when body fat is low or food is scarce. In the gut, Neuropeptide Y generally slows gastric emptying and transit time. |
| Serotonin | Released both in the brain and the enteric nervous system. In the brain, serotonin is linked to modulating anger, aggression, temperature, mood, sleep, appetite, and vomiting. Following meals, serotonin concentrations reach a maximum within 1-2 hours.In the gut, serotonin is produced by cells located in the small intestine. In this capacity, serotonin increases small intestinal motility, reduces stomach acid production, and, in high amounts, can cause nausea. This is why anti-depressant drugs like Prozac can sometimes lead to diarrhea and nausea. These drugs make more serotonin available not only in the brain (where they exert their anti-depressant effect), but the gut, where they can cause serotonin excess. |
| Nitric oxide and Substance P | Found in the brain and enteric circulation, these compounds are associated with vasodilation in the gut, assisting in more blood flow for nutrient delivery/uptake. |
| Vasoactive intestinal peptide (VIP) | VIP is important to the digestive process through its ability to inhibit the release of gastrin, inhibit the secretion of acid, stimulate bicarbonate secretion from the pancreas, induce smooth muscle relaxation and vasodilation, stimulate pepsinogen release, and stimulate the secretion of water and electrolytes into the small intestine. Most of these functions are responsible for slowing down stomach activity while stimulating intestinal activity. |
Other gut-brain interactions
Appetite medications
Sibutramine is the only regularly used anti-obesity medication. It acts as a norepinephrine-serotonin reuptake inhibitor. It causes hazardous side effects with only minimal impact on weight.
Rimonabant is a cannabinoid type 1 receptor antagonist. It isn’t on the market since it causes psychiatric disturbances and increased suicide risk. And really, as of now, obesity meds don’t help at all. Patients might lose 10-15% of body weight, but 6 months later the weight is back on.
Exercise
Physical activity plays an important role in appetite regulation. Some data show that appetite responses to exercise are strongly influenced by energy balance in men, but less in women.
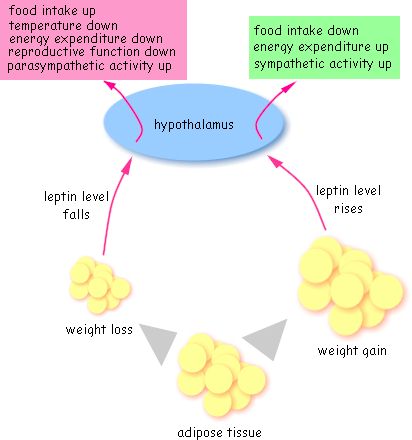
Those who regularly exercise become more efficient at using body fat as a fuel source, and this can help with regulating appetite (since people that don’t exercise use more carbs and blood sugar fluctuations = appetite swings). Exercise can moderate levels of leptin, ghrelin and insulin. (See leptin cycle below.)
Also, PYY 3-36 might increase with exercise, especially with stuff like walking, jogging and biking (rather than higher intensity stuff). Moderate to intense exercise transiently suppresses appetite.
Bariatric surgery
Many of the gut hormones and neurotransmitters mentioned seem to be affected (at least temporarily) after bariatric surgical procedures. This may promote satiety, at least in the short term.
Summary and recommendations
Eating a reasonable amount of food each day to support health and regulate appetite goes beyond willpower and calorie counting.
Acknowledging the information our body relays about hunger and fullness can be helpful in regulating appetite. Only when our physiological foundation is polluted with excess stress, weight, processed foods, and/or lack of physical activity will appetite balance become defective.
The appropriate release, response and balance of gut hormones and neurotransmitters seem to depend upon a diet consisting of whole foods.
While we don’t know exactly what it takes to manage appetite, we do know the human body doesn’t have a longstanding relationship with processed food products, and they might short-circuit our appetite regulation pathways.
Social rituals of eating, such as eating while distracted (e.g. while driving or watching TV), eating too rapidly (e.g. while rushing to do errands), or always having dessert may also affect our desire to override natural hunger and fullness cues.
Extra credit
Estrogen deficiency might result in a higher energy intake and increased body weight. Food intake varies across the menstrual cycle. Women tend to eat more in the luteal phase (the premenstrual period) compared with the follicular phase.
Testosterone (directly) seems to have little effect on food intake, although many people supplementing anabolic doses of testosterone (e.g. bodybuilders) do report increased appetite.
Including a balanced intake of omega-6:omega-3 fats can help with appetite regulation.
Protein and fibre all seem to help control appetite.
Refined carbohydrates, on the other hand, appear to increase appetite.
Dietary fat has mixed results; when combined with refined carbohydrate it seems to increase appetite while on its own or combined with protein, it typically decreases appetite.
Elderly people have less appetite than young people from not only decreased energy expenditure but also from mechanisms potentially involving sex–steroid balance as well as altered CNS signaling to and from peripheral organs.
It’s now recognized that overfat individuals have lower blood concentrations of vitamins and minerals compared to leaner individuals. This may lead to a greater appetite and changes in fat deposition.
References
Click here to view the information sources referenced in this article.
If you’re a coach, or you want to be…
You can help people build sustainable nutrition and lifestyle habits that will significantly improve their physical and mental health—while you make a great living doing what you love. We'll show you how.
If you’d like to learn more, consider the PN Level 1 Nutrition Coaching Certification. (You can enroll now at a big discount.)

Share