Some experts—and lots of people on Twitter—believe carbs and insulin make you gain weight. But, they say, the fix is simple: If you eat a low-carb diet, you’ll keep insulin levels low—and lose weight rapidly instead. All without ever having to worry about calories. Here, we look at the science behind these claims and try to answer the real question on everyone’s mind: What really matters most for fat loss?
+++
People used to call pasta “diet food.”
But over the last two decades, carb-phobia has sky-rocketed.
And now? Pasta is more commonly known as “fattening.”
So when folks want to lose weight, they’re often told to eliminate the rigatoni, rotini, and ravioli—along with rice, potatoes, bread, and even fruit.
The reason: Carbs, of course… and the hormone insulin.
It’s all based on a controversial hypothesis known as the carbohydrate-insulin model of obesity.
From 30,000 feet, it looks like this:
- You eat carbohydrates.
- Your body releases insulin.
- Then, according to the model, insulin 1) keeps your body from burning fat for energy, and 2) drives fat and sugar from your bloodstream into your fat cells.
- All this makes your body think it’s starving, causing it to slow your metabolism and increase your hunger.
It’s a beautifully simplistic explanation as to why we have a still-growing global obesity problem.
And many advocates of the carbohydrate-insulin model claim it leads to a beautifully simplistic solution: Adopt a low-carb diet.
With this approach, they say, you’ll create a hormonal environment that gives you a “metabolic advantage,” allowing you to effortlessly lose fat while eating as much as you want.
No more worrying about calories or portions.
(Want to get the world’s most useful nutrition, health, and coaching strategies delivered straight to your inbox? Sign up for our FREE weekly newsletter, The Smartest Coach in the Room.)
The question is: Does it hold up scientifically?
In this article, we’ll walk you through the science of how the carb-insulin relationship works—for both health and fat loss—and answer these questions:
- Does insulin stop you from burning fat?
- Does insulin cause weight gain?
- Does insulin make you hungrier?
- Does insulin decrease your metabolism?
- Do people lose more weight on low-carb diets?
- What matters most for fat loss?
(Fair warning: We’re going deep, so you may want to grab coffee.)
(If you want to see the authors discuss this article in even more detail, check out the video below. If not, simply scroll over the video player or click here to jump to the next section.)
PN coach roundtable: Robin Beier discusses the truth about insulin, carbs and weight loss with Helen Kolias and Brian St. Pierre.
Insulin and carbs: Partners in crime?
To fully understand the carbohydrate-insulin model, you have to start with some biology. (Skim at your own risk!)
So here we go…
When you eat certain carbohydrates—such as starch and sugar—they’re quickly broken down into glucose and absorbed into your bloodstream. This raises your blood glucose levels. (Also called blood sugar levels.)
The more carbohydrates you eat, the higher your blood glucose rises immediately after that meal.
Your body, however, strives to closely regulate your blood glucose levels.
Ever had your fasting glucose measured? You probably know the “normal” range is 70 to 100 mg/dl.
Your body wants to maintain this level of blood glucose, to keep you healthy and all systems functioning optimally.
(For example, chronically elevated blood glucose levels cause inflammation that can damage your blood vessels, kidneys, eyes, and nerves. This is why diabetes can lead to many health complications.)
Enter insulin.
When you eat carbohydrates, and blood glucose rises, your body—specifically your pancreas—releases insulin. That’s because insulin is your body’s key regulator of blood glucose.
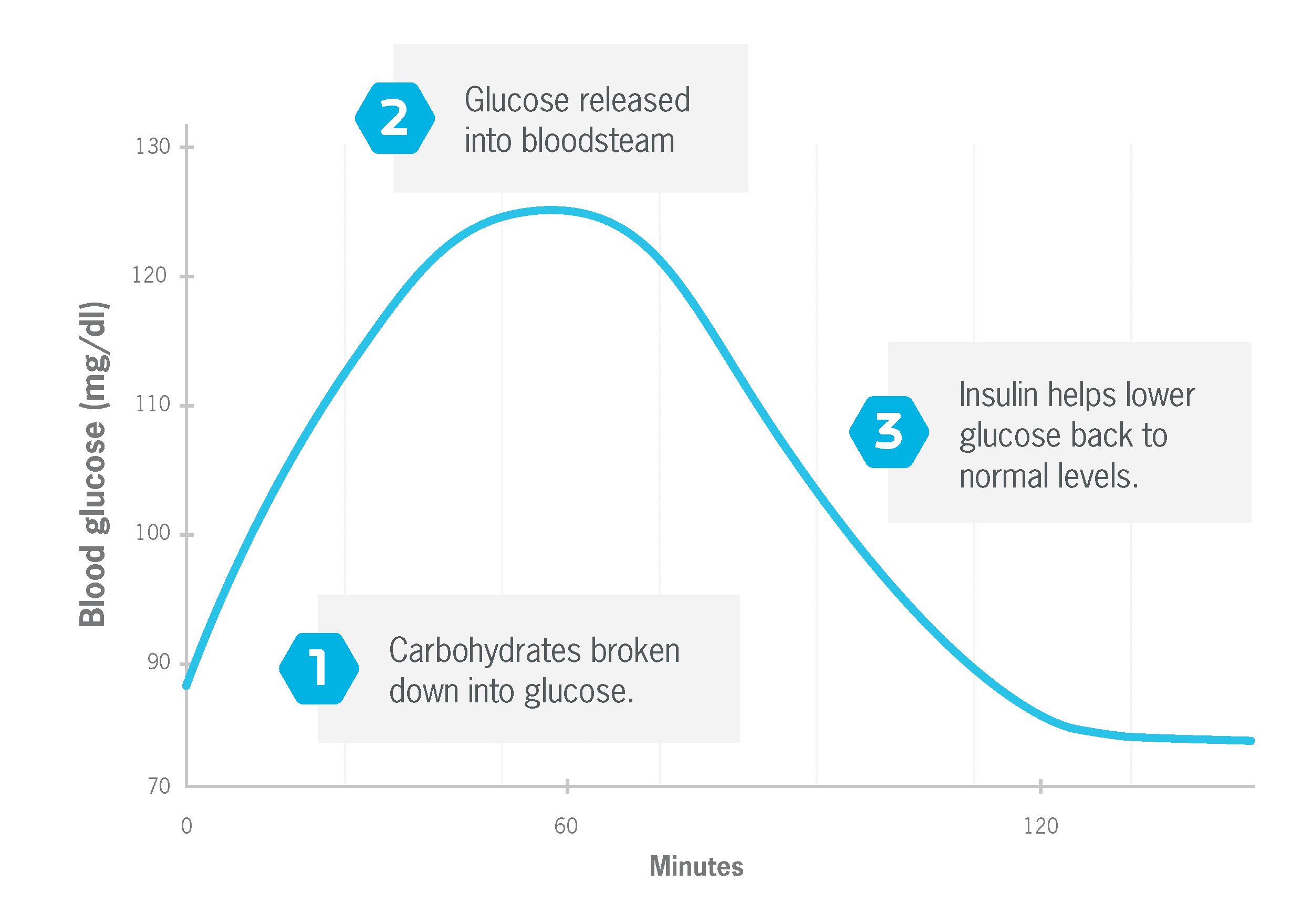
Insulin is needed to shuttle glucose from your blood into your muscle and fat cells, where it can be used for energy or stored for later use. 1
Without insulin, your blood glucose levels would stay elevated for a much longer period. And that would be very bad. This is why people with type 1 diabetes must take insulin every day via injections or a pump.
The bottom line? When blood glucose goes up, insulin goes up.
And remember: If you eat lots of carbs at a meal, your blood glucose and insulin levels go up more than if you eat fewer carbs.
Context matters, too. People respond differently to the same number of carbohydrates based on many factors, including 2,3,4:
- Fitness level
- Body fat
- Genetics
- Microbiome health
- Muscle mass
- How recently, vigorously, and long they’ve exercised
- Time of day
- What else they’re eating (for example, fat and fiber—another type of carbohydrate—can slow the absorption of glucose, blunting the insulin response, while certain proteins can increase the insulin response.)
Typically, the leaner and more active a person, the more sensitive their cells are to insulin. (Known as insulin sensitivity.) Meaning, they need less insulin to move glucose out of their bloodstream.
This is one reason why fit people “tolerate” carbs better than sedentary folks. They usually even benefit from more carbs, to aid performance and recovery.
Why insulin can be a problem
As we’ve noted, when your body is functioning normally, glucose and insulin are in lockstep. When blood glucose rises, just enough insulin is released to bring glucose back into the normal range.
But there’s also a scenario where you can have too much insulin. This is generally thought to happen when your cells become resistant to insulin, a condition known as insulin resistance, a precursor to type 2 diabetes.
With insulin resistance, a greater amount of insulin is needed to get the same amount of glucose into your cells. And as the condition worsens over time, insulin levels can remain elevated even when you haven’t eaten anything. (This is called hyperinsulinemia.)
We don’t know exactly why insulin resistance happens. It’s mainly thought to be caused by chronically-elevated levels of fatty acids in your bloodstream.5
(Insulin resistance is also related to a host of factors including genetics, ethnicity, sleep, exercise habits, smoking habits, and more.6,7,8)
What we do know is that people who are obese—particularly those with higher amounts of visceral fat (the deep abdominal fat that surrounds several vital organs)—are more likely to be insulin resistant.
We also know losing excess body fat often resolves insulin resistance.
Now that you have the background, let’s dive into the first question…
Does insulin stop you from burning fat?
Not exactly. But insulin does influence the rate your body burns fat.
That’s because, in addition to insulin’s role as the key regulator of blood glucose, it’s well-known that:
Insulin inhibits lipolysis.
During lipolysis [lie-PAWL-uh-siss], stored fatty acids are moved out of your fat cells and into your bloodstream, where they can be used for energy.
When this process is inhibited—as it is when insulin levels are high—fewer fatty acids are available to fuel your muscles and other metabolically active tissues. Because of this, many people equate insulin spikes with “turning off” your body’s ability to burn fat.
Insulin stimulates lipogenesis.
During lipogenesis [lie-POE-jen-uh-siss], fatty acids are moved from your bloodstream into your fat cells, where they’re stored for later use. This is often referred to as being in “fat storage mode”—something most people try to avoid.
What’s more, lipogenesis can also cause carbs to be converted to and stored as fat (known as de novo lipogenesis, or DNL). It’s important to note, though: DNL only happens in meaningful amounts when there’s an overall surplus of carbs and calories. (That is, you consistently eat more calories than you burn.9)
As a result of all these effects, you might conclude that insulin is a real problem for fat loss.
So it’s worth saying:
Insulin’s purpose isn’t to make you fat.
Insulin inhibits lipolysis because you just consumed nutrients, specifically carbohydrates and/or protein. And it’s more efficient for your body to use those incoming nutrients for energy than to liberate stored nutrients for energy.
Think of it this way: If you have $100 in your pocket, and you want to buy $25 in groceries, you wouldn’t go to the ATM for more cash. You’d use the money already in your pocket.
Similarly, why would your body release stored fat into your bloodstream for energy when there’s plenty of incoming energy already available?
Also, at any given time, there’s a complex interplay of hormones and enzymes that can counteract, limit, or enhance the effect of any single chemical, including insulin.
For instance, while insulin inhibits lipolysis (fat burning), other hormones—which are active at the same time—stimulate lipolysis.10 Examples:
- Glucagon
- Epinephrine
- Norepinephrine
- Growth hormone
- Cortisol
Furthermore, while insulin stimulates lipogenesis (fat storage), other active hormones—leptin, growth hormone, and acute increases in cortisol—inhibit lipogenesis.11
These hormones don’t completely disappear from your body in the presence of insulin. They have important jobs, too, and can modulate the effects of insulin.
For instance, while carbohydrate is the major macronutrient impacting insulin, protein also significantly stimulates insulin secretion.12,13 Yet protein is generally thought to contribute positively to body composition improvements.
Some hypothesize this is because protein also stimulates production of the hormone glucagon, thus negating the effect of insulin.14
Whatever the case, the impact of insulin on metabolism isn’t straightforward: It’s tempered by many other factors. (To read another example of this, check out “FGF-21: The “secret” metabolism hormone” below. Or you can skim over the box—or click here—to continue with the main article.)
FGF-21: The “secret” metabolism hormone
Clearly, insulin is the key mechanism of the carbohydrate-insulin model.
But without strong clinical evidence from controlled studies (we’ll dive into this more later), how can you support that mechanism?
Answer: You need a deep understanding of how all the other hormones and metabolic processes work together.
Otherwise, the model can’t reliably predict what will happen in every situation. Which makes it… an incomplete and thus unreliable model.
For example, part of the natural progression of type 2 diabetes is that insulin levels go down over time.15
Based on the carbohydrate-insulin model, this should make it easier for people who’ve had type 2 diabetes for years to lose weight, compared to someone who has pre-diabetes.
But we don’t see this. The pounds don’t suddenly fall off people after they’ve had type 2 diabetes for several years.
If we don’t understand why this contradiction occurs, how confident can we be that the carbohydrate-insulin model is correct?
The reality is this: You can’t just consider insulin. There are many other hormones involved in fat loss, appetite, hunger, and metabolism—plenty of which aren’t well understood.
Take, for example, fibroblast growth factor-21 (FGF-21). It’s thought to be an important regulator of whole-body metabolism and energy homeostasis, yet you’ll rarely hear anyone talk about it.
Research shows that FGF-2116,17:
- Decreases appetite
- Decreases the rate carbs are burned for energy
- Increases the rate fats are burned for energy
- Improves blood glucose control
- Increases brown fat activity (a metabolically active type of fat)
That’s a pretty strong resume.
Interestingly, eating excess carbohydrates increases FGF-21, but overeating fat doesn’t.18 And under certain conditions, FGF-21 can override insulin to stimulate lipolysis (fat burning).19
This isn’t to suggest that FGF-21 is some secret to fat loss. (Such a secret doesn’t exist.) But rather to ask the question: How does FGF-21 fit into the carbohydrate-insulin model?
Right now, it’s not clear. And that might mean it’s a faulty model.
Instead of thinking about the effects of insulin—or any of these hormones—as an on-off button, picture a dimmer switch.
Your body is constantly adjusting its hormonal dials, not based solely on food intake, but also on thousands of other inputs and processes you aren’t even aware of.
The upshot: When your insulin levels are high, you’ll burn less fat for energy than when your insulin levels are low. But you won’t stop burning fat altogether.
You’ll preferentially burn carbohydrates for energy instead.
So…
The real question isn’t whether insulin stops you from burning fat. It’s whether insulin stops you from losing fat.
Here’s what we can say with confidence: There’s zero scientific evidence to suggest you’ll gain weight if your energy intake is less than your energy expenditure. (Not counting short-term changes in body water, of course.)
Or put another way: Insulin itself doesn’t cause weight gain. You also need to eat more calories than you expend.
Remember, in healthy people, the increase in insulin after a meal only lasts a few hours. Then it returns to baseline, allowing fat burning to throttle up again.
If energy intake is lower than energy expenditure, insulin will stay low for long periods throughout the day and night. This permits fat burning to occur at full effect despite short periods of fat-burning inhibition.
So, if you initiate a diet to lose fat, you can accomplish that with or without carbs.20 (We’ll look at the research that compares the effectiveness of different diets in a moment.)
Does insulin make you hungrier?
One of the key positions of the carbohydrate-insulin model: High insulin levels—thanks to a high-carb diet—make you eat more.
But the evidence to support this assertion is weak.
Here’s the premise: Because insulin signals your body to store fat, it “empties” your bloodstream of fatty acids and glucose, shunting them to your fat cells.
It’s hypothesized that this triggers something termed “internal starvation.”14
By “emptying” your blood of these fatty acids and glucose, your brain thinks you’re starving. And this, in turn, drives you to eat more food.
But do the fatty acids in your bloodstream actually decrease?
As Stephan Guyenet, PhD points out, research shows people with obesity exhibit normal or even high levels of fatty acids in their bloodstream.21,22,23,24
What’s more, insulin has long been thought to help regulate appetite.25 It’s speculated, based on animal research, that elevated blood insulin levels signal your brain to reduce food intake. (This has been studied directly in primates but not in humans.)
So, in this model, elevated insulin would decrease the drive to eat.
But just like fat burning and fat storage, insulin isn’t the only hormone involved with appetite regulation. Others include26:
- Leptin
- Cholecystokinin (CCK)
- Ghrelin
- Amylin
- Glucagon-like peptide 1 (GLP-1)
And that’s just to name a few.
The point: Hunger and appetite regulation is incredibly complicated.
It’s not likely as simple as reducing insulin or adjusting any one factor.
Which brings us back to the original question: Does the hormone insulin make people hungrier?
There’s no strong physiological evidence that it does. In fact, a new highly-controlled study—which we’ll discuss later in this article—presents data that’s in conflict with this assertion.
Plus, competing mechanisms strongly suggest other factors, such as the hormone leptin, may be of much greater importance than insulin. (To read more about the role of leptin, check out: Eating Too Much? Blame Your Brain.)
Does insulin decrease your metabolism?
Metabolism is highly related to body size. People with larger bodies generally have higher resting metabolic rates than people with smaller bodies.27
So, when people lose weight, their metabolic rate decreases. But typically, this reduction is even greater than what you’d expect from the change in body mass alone.27
This is known as metabolic adaptation (which also seems to be largely driven by leptin), and it’s perhaps one reason it’s hard to sustain weight loss. Your body requires fewer calories to maintain your new weight than someone who’s been that same weight most of their adult life.
According to the carbohydrate-insulin model, high-carb diets—and elevated insulin levels—are responsible for this metabolic adaptation.14
The hypothesis: Because insulin directs fatty acids out of the blood—toward fat cells and away from more metabolically active tissues like muscle—the result is a decreased metabolic rate.
This, however, is in conflict with research that shows insulin increases fatty acid uptake in muscle.28
On the flip side, the hypothesis proposes that low-carb diets—due to their insulin-lowering effect—provide more fuel for metabolically active tissue. This keeps your metabolism stoked, like throwing wood on a fire.
And it’s what advocates for the carbohydrate-insulin model term a “metabolic advantage.”
But is this really what happens? Does a low-carb diet actually increase your metabolism compared to a high-carb diet?
Let’s see what human studies can tell us.
What does diet and metabolism research say?
The most in-depth look into this topic is a 2017 meta-analysis led by Kevin Hall, PhD at the National Institute of Diabetes and Digestive and Kidney Diseases (an institute of the NIH).27
The researchers examined 32 calorie-matched, controlled-feeding studies that directly compared low-carb and high-carb diets and their effects on daily energy expenditure.
“Calorie-matched, controlled-feeding” means both diets contained the same number of calories, and the scientists provided all food to the participants.
These studies also matched protein amounts between diets.
This is important because protein requires more calories to digest (25 to 30 percent) than both carbohydrate (6 to 8 percent) and fat (2 to 3 percent).29
If one diet were to include a substantially greater amount of protein, energy expenditure would likely be higher, regardless of carb intake.
What did the data show?
Energy expenditure was 26 Calories higher per day in the high-carb diets versus the low-carb diets.
This conclusion, however, has been criticized by David Ludwig, MD, PhD, a leading proponent of the carbohydrate-insulin model.
That’s because only four of 32 of the studies had durations of at least 2.5 weeks, and according to Dr. Ludwig, it takes two to three weeks for the body to adapt to a low-carb diet, also known as being “fat-adapted.”14,30,31,32,33
Currently, there’s no validated method for objectively measuring if someone is fat-adapted. So while it may indeed take longer than two weeks, no one knows if that’s true or can say how they know when it occurs.
To support their assertion, however, proponents of the carbohydrate-insulin model often cite the results of a 20-week study from Dr. Ludwig’s group, conducted after Dr. Hall’s 2017 meta-analysis.34
A breakthrough study?
In a 2018 study, Cara Ebbeling, PhD, Dr. Ludwig, and their research team first had study participants lose 10.5 percent of their weight by adhering to a calorie-restricted, 45 percent carbohydrate diet for 9-10 weeks. The successful dieters then followed a 20-week maintenance diet that was either:
- Low carbohydrate (20 percent)
- Moderate carbohydrate (40 percent)
- High carbohydrate (60 percent)
The results:
- Low-carb dieters expended 278 Calories more per day than high-carb dieters.
- Moderate-carb dieters burned 131 Calories more per day than high-carb dieters.
- It’s also worth noting that the participants successfully lost an impressive amount of weight prior to adopting the low-carb diet. They dropped an average of 21 pounds in the initial 9-10 week while consuming 45 percent of their calories from carbs.
At the time, it was the best evidence to date that low-carb diets may offer a significant metabolic advantage. (Keep reading for the latest study.)
But it’s also faced intense scrutiny from Dr. Hall and other experts, who’ve questioned the measurement and reporting methods that were used, as well as the statistical analysis.35
And because the study participants were living in their normal environment—not in a lab—it’s possible not all food intake was accounted for.
There’s also this: If low-carb diets truly have a metabolic advantage, people should lose more fat than those on higher carb diets. Dr. Hall’s meta-analysis didn’t show that. In fact, it showed the opposite (by a tiny amount).
But let’s dig deeper into the research.
Do people lose more weight on low-carb diets?
Yes? No? Maybe? Sometimes?
In many studies—ranging from a few weeks to several months—low-carb diets have often outperformed high-carb diets.36,37,38,39,40,41
But is this specifically due to a metabolic advantage? Or do low-carb diets offer other benefits?
One popular and logical explanation is that people eat fewer calories on a low-carb diet versus a high-carb diet.
Most studies that show a low-carb diet leads to greater weight loss aren’t “protein-matched, calorie-matched, controlled-feeding studies.”
Instead, they frequently provide dietary counseling and menus to participants, advising them what to eat, but not monitoring food intake closely.
This is a downside in terms of observing the specific effects of each diet. But it could be a positive when looking at how these diets work in everyday life.
After all, this is how the average person follows a diet plan.
Why might a low-carbohydrate diet cause people to eat less? There are a few potential reasons:
- Greater intake of protein increases satiety and reduces appetite42
- Limited food choices cut out hundreds of highly-processed calories they might have eaten otherwise—such as cookies, muffins, and chips—and made room for more nutrient-dense and calorie-sparse foods like produce
- Reduced food options can also lead to “sensory-specific satiety.” Meaning, when you eat the same foods all the time, they may become less appealing, so you’re not driven to eat as much43
- Liquid calories—soda, juice, even milk—are generally off-limits, so a greater proportion of calories are consumed from solid foods, which are more filling44,45,46
- Higher blood levels of ketones—which rise when carbs are restricted—may help to suppress appetite47,48,49
All of which sounds pretty ideal (but still speculation).
There’s a problem, though: Over time, adherence to energy-restricted low-carb diets wanes, just like it does with other diets. So much so, that after a year, weight loss (and fat loss) tends to be either underwhelming or not significantly different between low-carb and low-fat diets.39,40,41
(Plus, 12-month studies on both low-carb and low-fat diets show that participants tend to shift to a more balanced diet over time.)
That’s not a knock on low-carb diets. Instead, it speaks to the difficulty that most people have of maintaining any restrictive eating approach for an extended timeframe.
But while these studies give us an idea of what happens in a free-living environment, they don’t provide a lot of insight into what happens physiologically under highly-controlled conditions.
The best research we have for that? Two metabolic ward studies conducted by Dr. Hall, published in 2016 and 2020, respectively.31,50
Meet the gold standard
Metabolic ward studies require participants to stay onsite for the duration of the trial. As a result, they’re the gold standard for human nutrition research.
The first study worked like this31:
- 17 male participants lived in a metabolic ward for two months. Everything they ate and how they lived were under strict control.
- First, they spent 4 weeks following a high-carb diet.
- Then, they spent 4 weeks on a very-low-carb ketogenic diet.
- With both diets, calories and protein were the same. Only carbs and fat went up or down.
- The diets created a negative energy balance of 300 Calories per day.
- Each participant had to do 90 minutes of stationary cycling per day to make sure physical activity levels were consistent and equal.
If the carbohydrate-insulin model were true, here are the results you’d expect to see:
- A drop in insulin output during the low-carb phase
- A significant increase in energy expenditure during the low-carb phase
- More fat loss on the low-carb diet than the high-carb diet
What the study found
| THE RESULTS | ||
|---|---|---|
| Low Carb (High Fat) | High Carb (Low Fat) | |
| Insulin | People produced 22% less insulin throughout the day | No change in insulin output |
| Energy Expenditure | An increase of 57 (+/- 13) Calories per day | No measurable effect |
| Weight loss | On average, 4 pounds lost, 1.16 pounds from body fat | On average, 3 pounds lost, 1.29 pounds from body fat |
So what does this mean?
- People lost the same amount of weight and fat (statistically speaking) on both diets.
- Though people produced less insulin on the low-carb diet, it didn’t result in significantly greater weight or fat loss.
- A slight increase in daily expenditure was observed, which supports the notion that low-carb diets may offer a small metabolic advantage during weight loss.
A study of “extreme” diets
The more recent study, pre-published in May of 2020 (and not yet officially peer-reviewed), took a slightly different approach and provides new insights worth exploring.50
Again, the researchers compared low carb versus high carb. But this time they examined even more “extreme” versions of the diets.
- An animal-based, low-carb diet (a.k.a. ketogenic diet)
74.6% fat, 9.9% carbs, 15.5% protein - A plant-based low-fat diet (a.k.a. vegan diet)
75.5% carbs, 10.5% fat, 14% protein
Both diets emphasized minimally-processed foods.
And, as the researchers note in the paper, the diets were more akin to the “exemplary” type of diets that health experts often recommend.
Important note: This wasn’t a weight-loss study.
Instead, the scientists randomly assigned 20 overfat participants (11 men and 9 women) to one diet for two weeks and then had them switch to the other for two weeks.
For each diet, participants were given three meals plus snacks per day, carefully prepared to provide twice the number of calories each individual required. The dieters were then told to eat as much or as little as they desired.
What the study found
► People ate 544 fewer daily calories on the plant-based low-fat diet than they did on the animal-based low-carb diet. (This data is only from the second week of each diet, to allow participants time to adapt. For both weeks combined, the difference was even greater: 689 fewer daily calories.)
► Energy expenditure was 166 Calories per day higher on the animal-based low-carb diet compared to the plant-based low-fat diet.
► Glucose and insulin levels were substantially lower during the animal-based low-carb diet.
► Participants rated both diets the same in terms of pleasantness and familiarity. So one wasn’t deemed more palatable than the other.
► They also reported no differences in satisfaction, fullness, or eating capacity, even though they ate significantly fewer calories on the plant-based low-fat diet.
► Both groups lost weight without intentionally restricting food intake: 3.9 pounds during the animal-based low-carb diet; 2.4 pounds during the plant-based low-fat diet.
► Only the plant-based low-fat diet (1.3 pounds) resulted in a significant reduction of body fat. The animal-based low-carb diet showed a significant decrease (3.5 pounds) in fat-free mass, most likely from water and glycogen, but this measurement also includes muscle, bones, and organs.
So what does this mean?
It shows an animal-based low-carb diet may offer a metabolic advantage, but that a plant-based low-fat diet may confer different advantages. Namely, people ate a lot fewer calories (though not necessarily less food) while reporting that they felt just as satisfied.
But instead of just looking at the differences, consider the commonality:
Participants literally ate as much as they wanted and didn’t gain weight on either diet.
Granted, both of these metabolic ward studies were very small and short term. While that’s a limitation, there’s a good reason for it: Imagine the challenge and expense of getting people to voluntarily live in a metabolic ward for up to two months, let alone six months or a year. (Maybe you don’t have to imagine, given the 2020 pandemic.)
What these studies do give you, though, is quality data, acquired in a highly-controlled environment, to consider for yourself.
Because no one has the “right” answer. We just have a body of evidence that we each have to weigh for ourselves.
Which brings us to perhaps the most important question.
What matters most for fat loss?
No matter if you eschew carbs or eat lots of them, there’s one thing for sure: You can’t separate a calorie from its food source.
Soda contains sugar. So does an apple. Both foods are mostly carbs.
But you can’t eat that apple without also getting some fiber, which slows the absorption of the sugar into your bloodstream. Plus, it’s a solid food that’s dense with other healthful nutrients.
What’s more, an apple isn’t highly-palatable or highly-rewarding, so it doesn’t stimulate your brain towards overconsumption like soda does. (To learn more, read: Manufactured deliciousness: Why you can’t stop overeating.)
All those factors affect fullness and food consumption.
Consider: A large Coke from McDonald’s provides 80 grams of sugar and 290 Calories. It’s relatively easy to consume in one sitting… along with a cheeseburger and fries, too.
But you’d have to eat four small apples (or 2.5 large apples) to consume an equal amount of sugar and calories from that soda. Know anyone that typically does that in one sitting? Or regularly wants to, even though they may thoroughly enjoy apples?
(And if you do, can we agree they’re an outlier?)
Same number of calories. Same amount of sugar. But a very different experience nutrition-wise.
How might this play out across your entire diet?
Dr. Hall conducted a study to gain insight.51
He admitted 20 adults to the NIH’s metabolic ward and randomized them to a diet of ultra-processed foods or minimally-processed foods. They were allowed to consume as much or as little as desired. After two weeks, they switched and did the alternative diet for two weeks.
The result: As you can see in the chart below, participants ate 508 more Calories per day and gained weight on the ultra-processed diet. They lost weight on the minimally-processed diet.
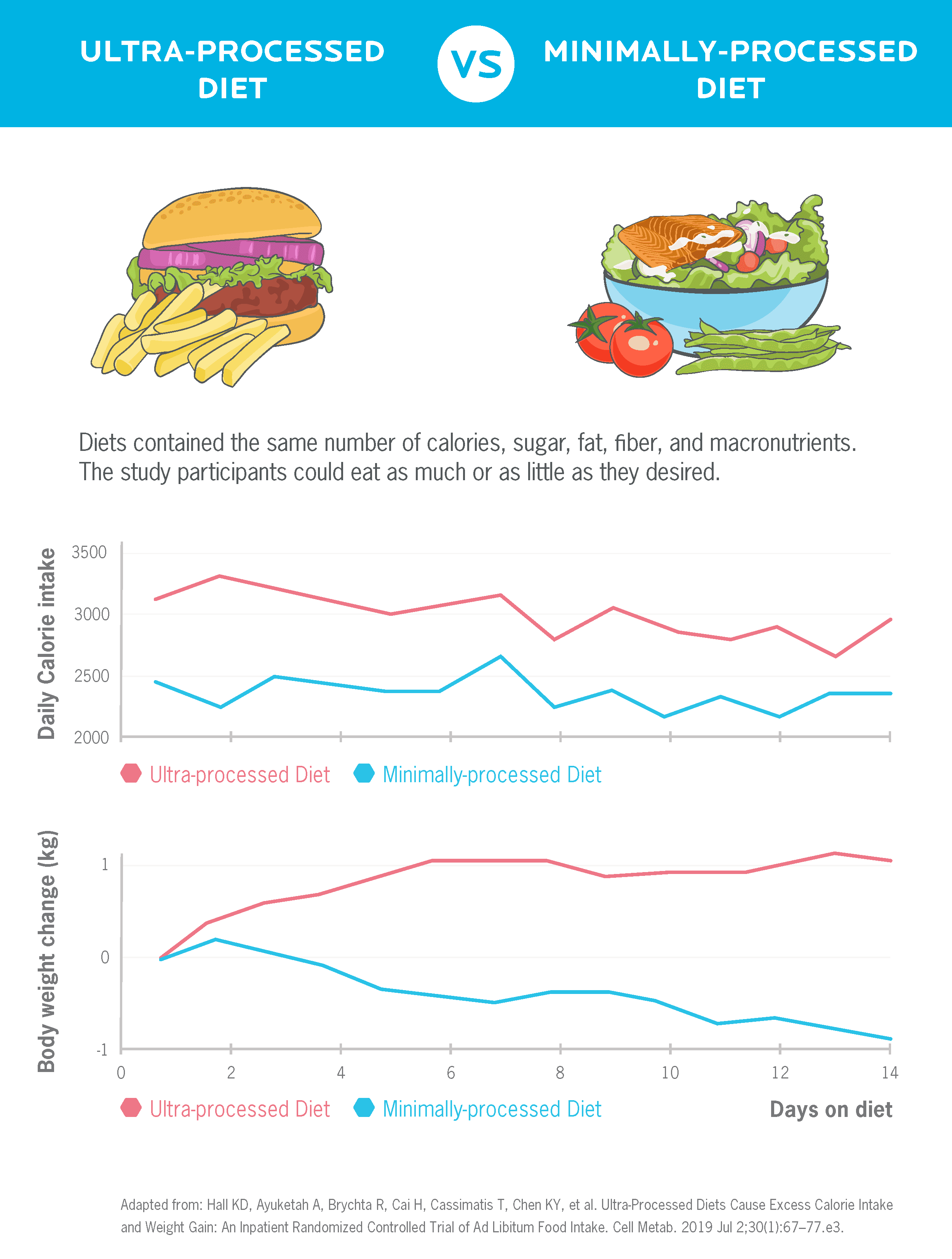
This probably isn’t shocking, but it’s illustrative of how the quality of foods we eat may have a greater impact on our weight than whether we cut carbs or fat. Further, it suggests that quality foods may make it easier to lose weight, without worrying so much about calories or hormones.
In his paper, Dr. Hall characterizes ultra-processed foods as being “typically high in calories, salt, sugar, and fat” and “engineered to have supernormal appetitive properties.”
Not surprisingly, people often refer to these types of foods as “addictive.” (Remember the “you can’t eat just one” slogan from Lay’s potato chips?)
Interestingly, a recent study from the University of Michigan looked at the “addictive” qualities of common foods.52
Take a look at the chart below. It shows the 10 foods that people are most likely to rate as “problematic,” using the Yale Food Addiction Scale.
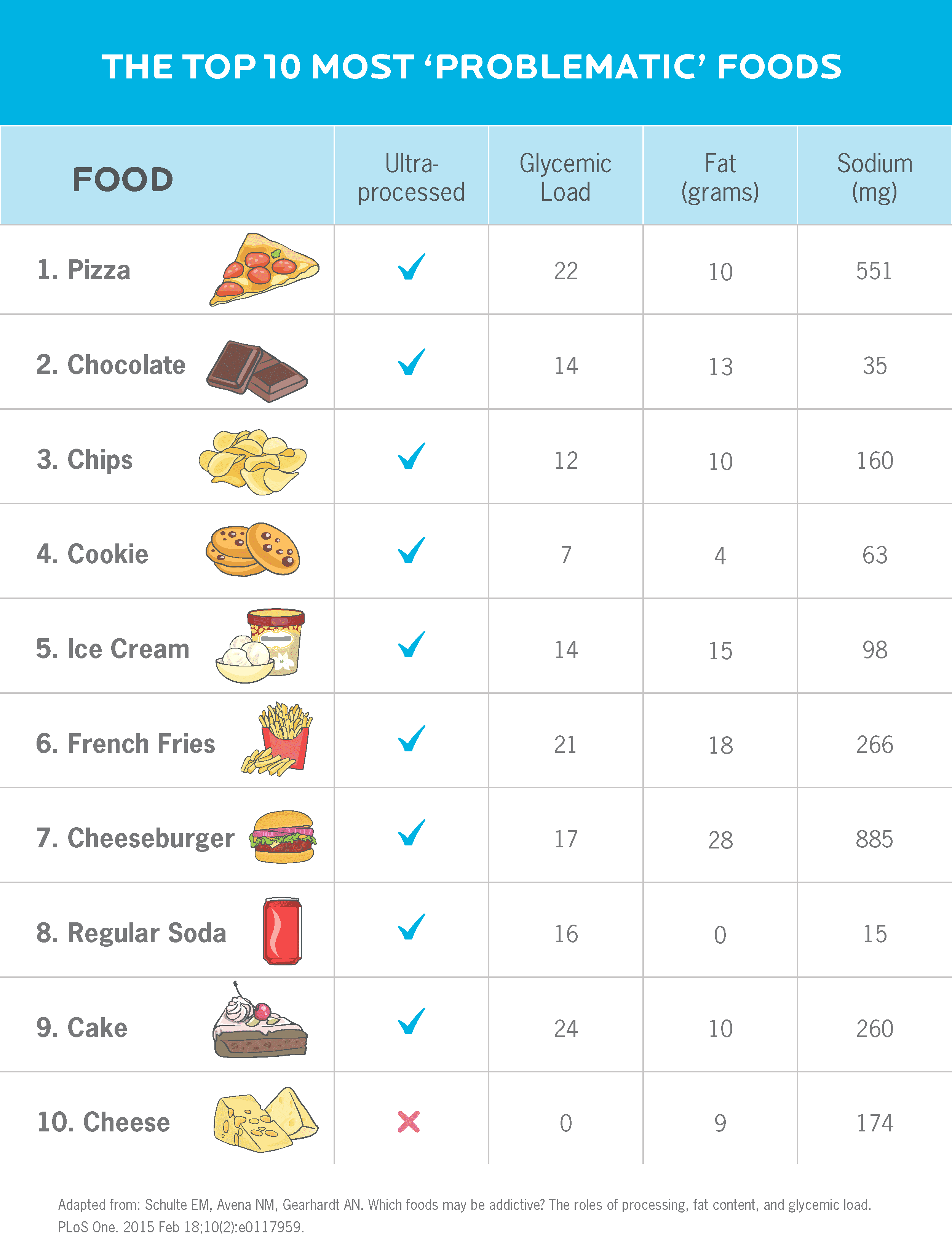
Note that all but one are ultra-processed foods, and most contain some combination of salt, sugar, and fat.
What about the foods, such as soda, that don’t have all three of those ingredients? They tend to contain “drug-like” compounds—such as caffeine and/or theobromine—to enhance their appeal.
Now consider: What foods do you (or your clients) feel are problematic? And what do they have in common?
Likewise, what foods aren’t problematic? That is, foods that you enjoy but can stop eating without overdoing it.
Perhaps an apple? Or salmon or cucumbers or beans? These types of minimally-processed foods all rated low on the scale.
(To test this on yourself or with a client, download our Yale Food Addiction Scale worksheet.)
And carbohydrate percentages aside, simply prioritizing whole foods aligns pretty closely with what low-carb advocate Dr. Ludwig recommends. From his recent paper14:
| Dietary Recommendations Based on the Carbohydrate-Insulin Model |
|---|
| Reduce refined grains, potato products, and added sugars—high glycemic load carbohydrates with low overall nutritional quality |
| Emphasize low glycemic load carbohydrates, including non-starchy vegetables, legumes, and non-tropical whole fruits |
| When consuming grain products, choose whole kernel or traditionally processed alternatives (whole barley, quinoa, traditionally fermented sourdough made from stone-ground flour) |
| Increase nuts, seeds, avocado, olive oil, and other healthful high-fat foods |
| Maintain an adequate, but not high, intake of protein, including from plant sources |
Emphasizing minimally-processed whole foods also seems to lead to better health. For example, in a recent Harvard University study, researchers looked at the effects of eating both “healthy” and “unhealthy” diets on all-cause mortality.53
Their findings: Consuming more minimally-processed foods, perhaps not surprisingly, was associated with greater longevity.
So ultimately…
It doesn’t matter what you believe about insulin, carbohydrates, or fat.
That might sound extreme, but what you believe doesn’t change what’s needed to lose fat and keep it off (or help a client do so):
- Eat less energy than you expend
- Develop eating, exercise, and stress-management habits that are sustainable long-term
If a low-carb diet helps you do that, great.
If a low-fat diet helps you do that, right on.
If a diet with a relatively equal balance of carbs, fat, and protein suits you better, that works too.
Paleo, plant-based, Meditteranean, keto, you name it: They’re all viable and can be effective, depending on your personal preferences, lifestyle, and needs.
What to do next…
Look at the big picture.
Obesity and weight gain are multifactorial.
Body fat is absolutely impacted by the kinds of foods you eat, your activity level, and, yes, your hormones.
But humans aren’t robots.
We have to look beyond just physiology and recognize that body fat is also influenced by many other factors, including:
- Social: stigma around fatness and peer pressure to eat a certain way
- Economic: the cost of food and exercise, and the pressure to perform at work (which can contribute to a lack of time to eat healthfully and exercise)
- Media: exposure to food advertising, how bodies are portrayed in the media, and availability of passive entertainment options (think: whether or not you have a Netflix subscription)
- Infrastructure: the walkability of your living environment, access to outdoor spaces, and whether your job is sedentary or physically active
- Medical: medications you may be taking, diseases you’re dealing with, or complications from past surgeries
- Developmental: how important food and exercise were in your family growing up, and the mindset you were raised with
While it’s comforting to think there’s one simple answer, it’s just not realistic.
Losing fat is likely to take a series of small steps to get where you want to go. Our advice: Focus on the “big rocks” before you worry about specific eating styles, nutrient timing, and supplements.
Big rocks include:
- choosing mostly minimally-processed, nutrient-dense foods
- eating enough lean protein and vegetables
- getting adequate sleep
- managing stress
- moving regularly
- reducing excessive smoking/alcohol consumption
The big rocks work for just about any diet approach you prefer.
By making the first few diet and lifestyle changes around these fundamentals, you can ensure that the changes you (or your client) make provide the most return on the effort.
Be open to testing your hypothesis.
Whether you’re already following a diet or eating style in pursuit of fat loss, or you have a specific one in mind, know that what works best for you might not be the thing you expect.
So no matter where you are in the process, put your scientist hat on and collect some data.
Ask yourself:
“How’s this diet working for me?”
Some signs it might not be working for you include:
- Difficulty staying consistent
- Frequently “falling off the wagon”
- Feeling tired, hungry, and/or cranky most of the time
- Not seeing results
- Avoiding social obligations because it’s too difficult to avoid temptation
If any of these resonate, be open to the idea that another approach might get you better results. (Download our Diet Satisfaction Assessment for a complete questionnaire that can provide insights.)
Remember that there’s no “best diet.”
There’s only what works best for you. And that can change over time.
A universal, one-size-fits-all, miracle diet would make good nutrition simpler. Unfortunately, it doesn’t exist.
What matters most for fat loss—and any other health pursuit—is finding an eating pattern that feels reasonable, sustainable, and yes, enjoyable.
And surely that’s a model that everyone can agree on.
References
Click here to view the information sources referenced in this article.
If you’re a coach, or you want to be…
You can help people build sustainable nutrition and lifestyle habits that will significantly improve their physical and mental health—while you make a great living doing what you love. We'll show you how.
If you’d like to learn more, consider the PN Level 1 Nutrition Coaching Certification.




Share